We are in the starting blocks to attend COMPAMED 2023 – one of the world’s most prestigious medical technology trade shows. This show is invaluable to us as it gives us the opportunity to experience first-hand the latest technological developments and innovations in our industry.
CompAMED functions not only as a showcase for technologies, but also as a platform for intensive professional exchange. We eagerly look forward to this opportunity to share our insights and explore potential collaborations with leading companies and professionals.
Our dedicated experts will be on hand during the show to identify groundbreaking solutions and products that can enrich our ongoing projects. Our top priority is to keep our finger on the pulse to provide world-class services and products to our customers.
We invite you to contact us to arrange a personal meeting and learn more about our innovations and services. Take this chance to explore common opportunities and discuss our cooperation. We are highly motivated to understand your needs and offer customized solutions.
Contact us today at mail@velixx.com, and we look forward to meeting you in person at COMPAMED 2023 from 13.11.2023 to 16.11.2023.

Our booth at MedtecLIVE with T4M in Nuremberg was a great success and an excellent opportunity for interesting discussions and inspiring new ideas.
Of course, anyone who was unable to visit us at our booth in person missed out. But we all know the challenges that can come with limited time and busy calendars. Sometimes you just can’t get everything done that you set out to do.

But luckily, you don’t have to wait until MedtecLIVE 2024 in Stuttgart to get to know velixX GmbH!
At velixX, our experienced engineers bring their expertise and professional knowledge to successfully implement your ideas. We offer customized solutions and individual consulting for your specific challenges. Through our large network of experts, your ideas can thrive and grow with us.
Our dedicated project managers are ready to drive your development and create customized solutions for you. You can count on us to thoroughly understand your requirements and achieve your goals.
If you are wondering how you can benefit from our expertise and services, please do not hesitate to contact us anytime at mail@velixx.com.
We are always ready for networking in medical technology and perfect healthcare solutions – and we look forward to hearing from you and developing innovative solutions together!
Wir sind stets bereit für Networking in der Medizintechnik und perfekte Gesundheitslösungen – und wir freuen uns darauf, von Ihnen zu hören und gemeinsam innovative Lösungen zu entwickeln!
The healthcare industry is constantly evolving, and as such, there is an ever-increasing demand for innovative solutions. At velixX GmbH, we believe that our approach to development is the key to providing perfect healthcare solutions that meet the needs of our clients.
We are excited to announce that we will be showcasing our innovative approach at the upcoming MedtecLIVE with T4M in Nuremberg from 23 to 25 May 2023. This is the most important trade fair of the year for us, and we are thrilled to be participating.

At velixX, we prioritize the expertise of our engineers, who take on the project leadership role. However, our development approach also involves a coordinated network of the best available development partners. This collaboration enables us to take on projects with large capacity reserves, offering swift and effective solutions.
Our flexibility extends to the involvement of universities, institutes, and renowned experts, which allows us to accompany all development steps, from feasibility studies to production.
At MedtecLIVE, we look forward to sharing our approach with you in person. We believe that our tailored approach and extensive expert network can help drive your healthcare project forward, and we would be delighted to discuss it with you over a cup of coffee.
If you would like to schedule a meeting, please contact us at mail@velixx.com.
At velixX, we are committed to delivering perfect healthcare solutions that meet your unique needs. Join us at the MedtecLIVE to learn more about how we can help you achieve your healthcare goals.
What happens when experts share their expertise and work together to develop innovative solutions? This is exactly the approach of the Healthcare Shapers network.

As a company committed to providing the best possible solutions for its healthcare customers, velixX believes it is important to be part of a network of professionals working toward the same goals.
What makes Healthcare Shapers special is that it brings together experts from the medical technology industry who are normally perceived as competitors. They work together to share their knowledge and drive projects forward together.
Healthcare Shapers network members recognize that the power of collaboration ultimately benefits the entire healthcare industry by driving innovation faster and improving patient outcomes.

The network’s annual conference provides an excellent opportunity to build and maintain personal relationships.
velixX CEO Manfred Augstein is proud to be part of this distinguished group of experts: “I am convinced that the network will provide valuable insights that we can incorporate into our own work at velixX GmbH to help our customers even more efficiently.”
Learn more about the type of contribution velixX can make to your project under Insights.
Go to Healthcare Shapers here.
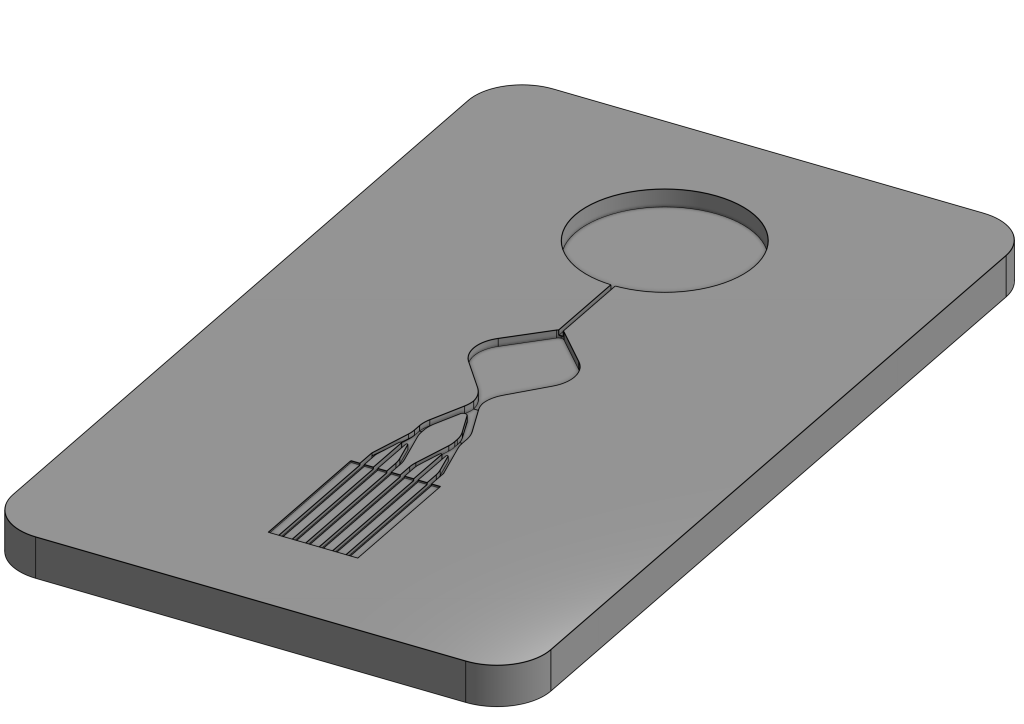

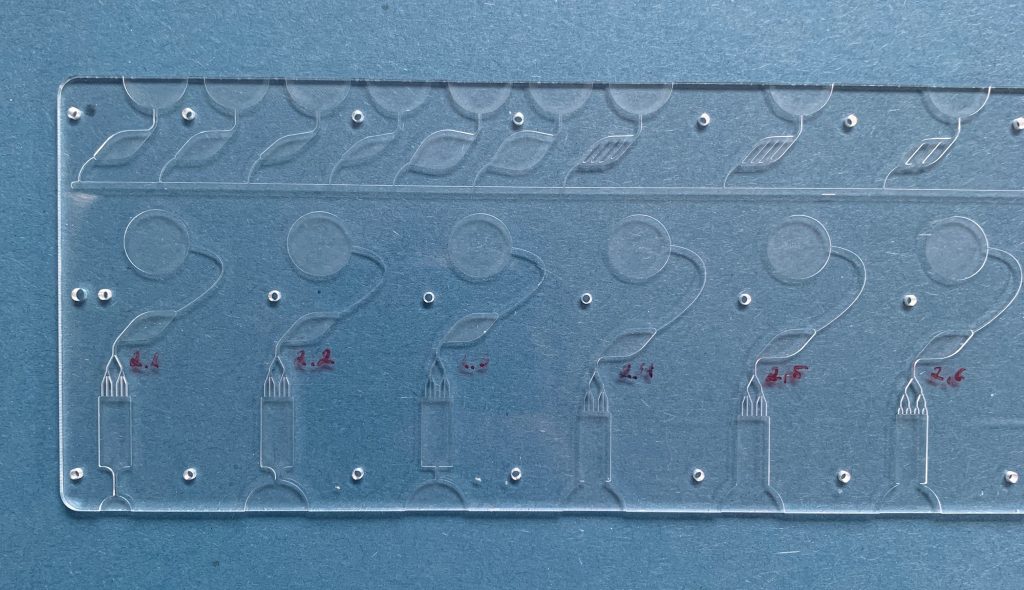
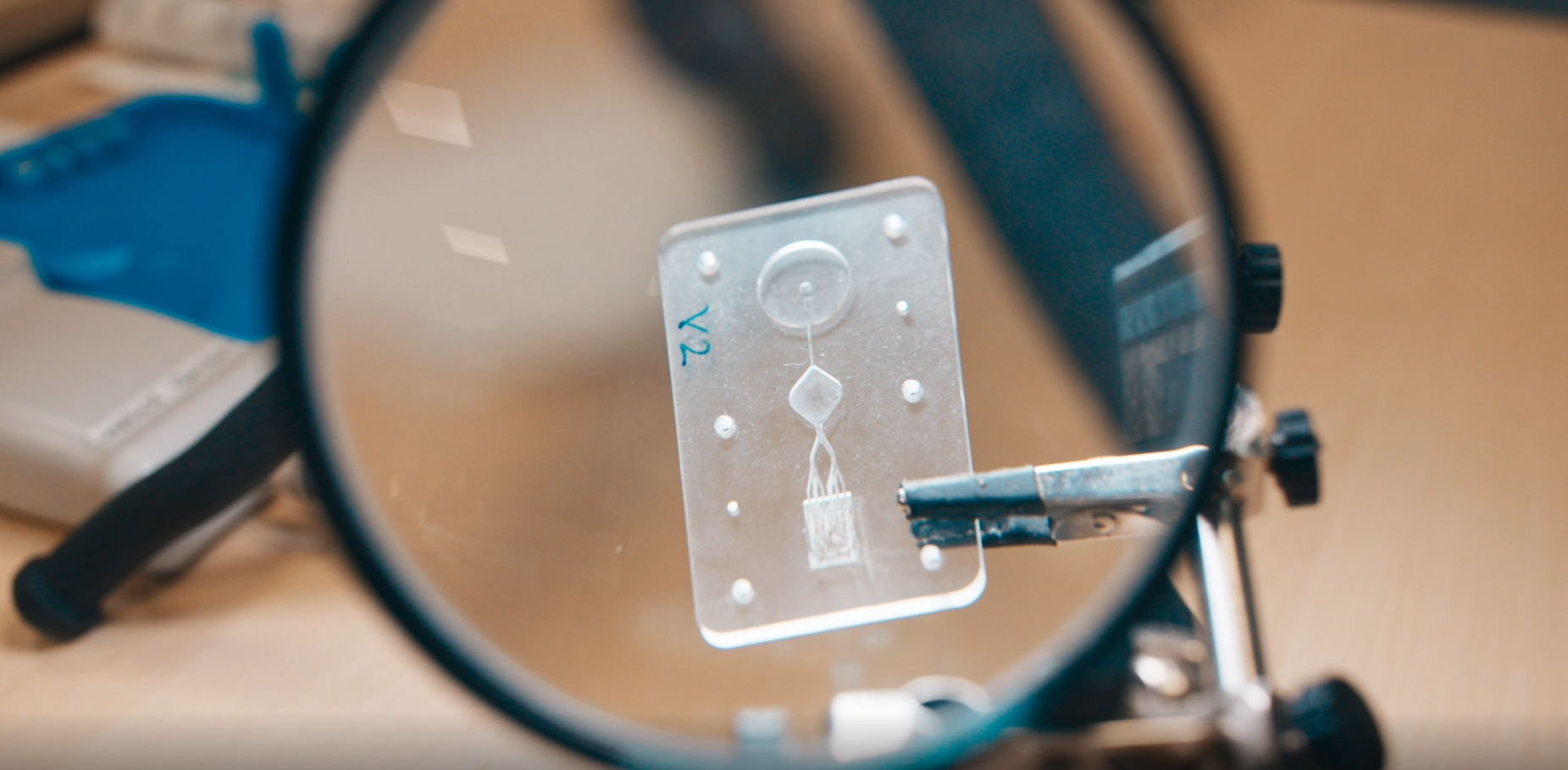
From microfluidic cartridges to complete lab-on-chip solutions – combining many functions in a very small space is one of velixX’ strengths. On behalf of our customers and in cooperation with our partners, we develop complex solutions in the smallest spaces.

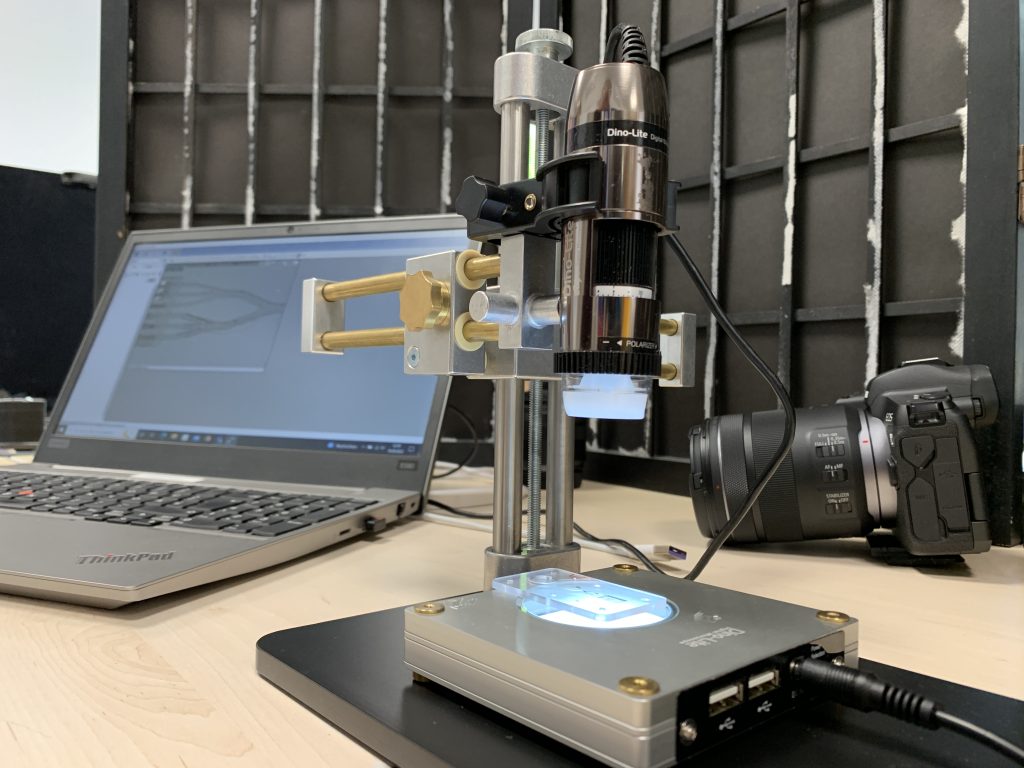
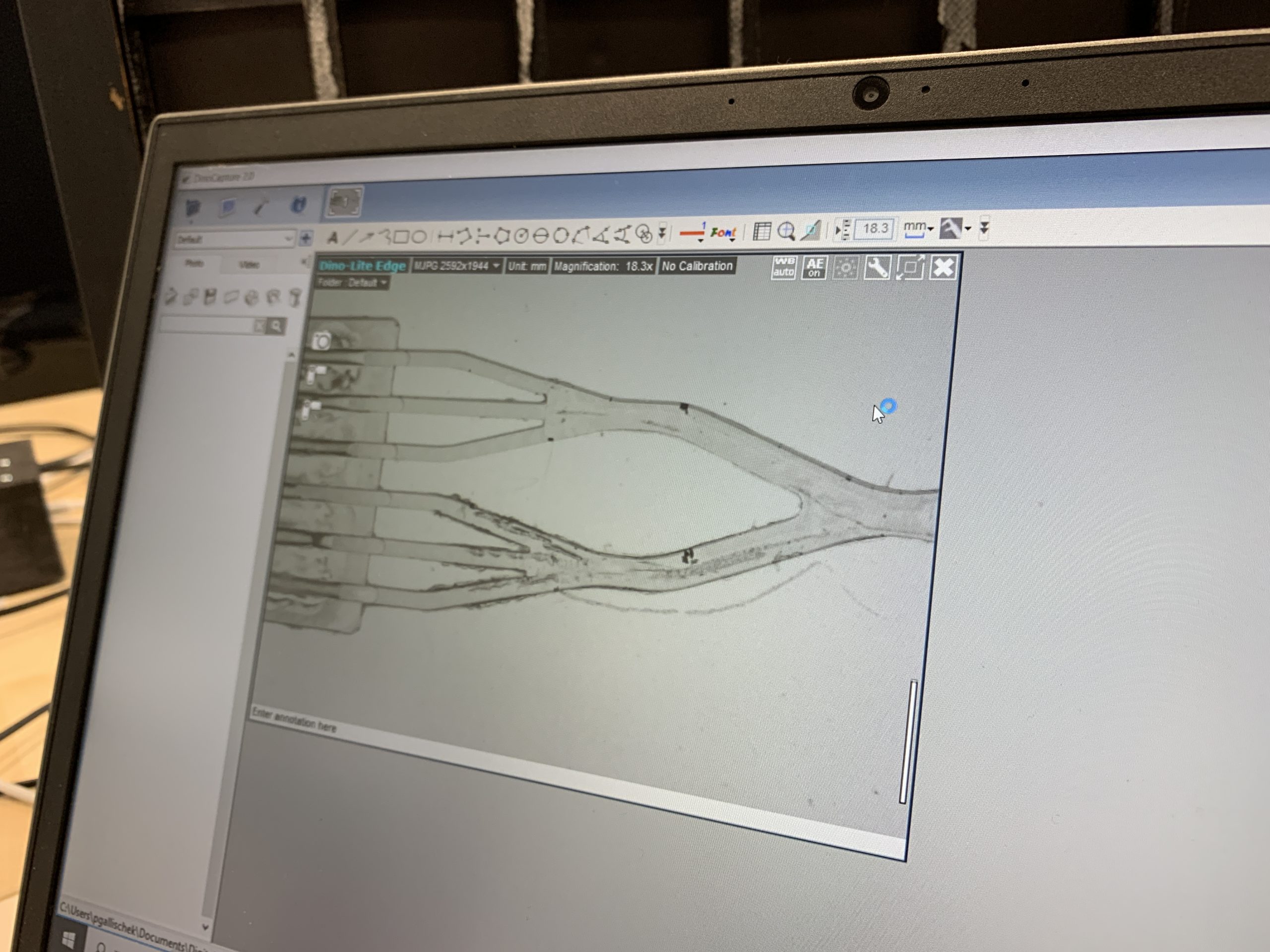
During the development process, we are able to implement many steps directly in-house. From the conception in CAD to 3D printing of initial designs to the milling machine for the creation of sample cartridges or the modification of microfluidic channels – our infrastructure allows us to react quickly, to test new ideas directly, and to make minor adjustments even without our external injection molding partners.
If we have awakened your interest and you would like to get to know us personally, please contact us at mail@velixx.com.



Numerous new projects with an increasing shift in focus usually lead to adjustments in a company’s infrastructure. Since microfluidics and in-vitro diagnostics have become increasingly important for velixX in recent months, it was now time to set up a separate room as a wet lab.

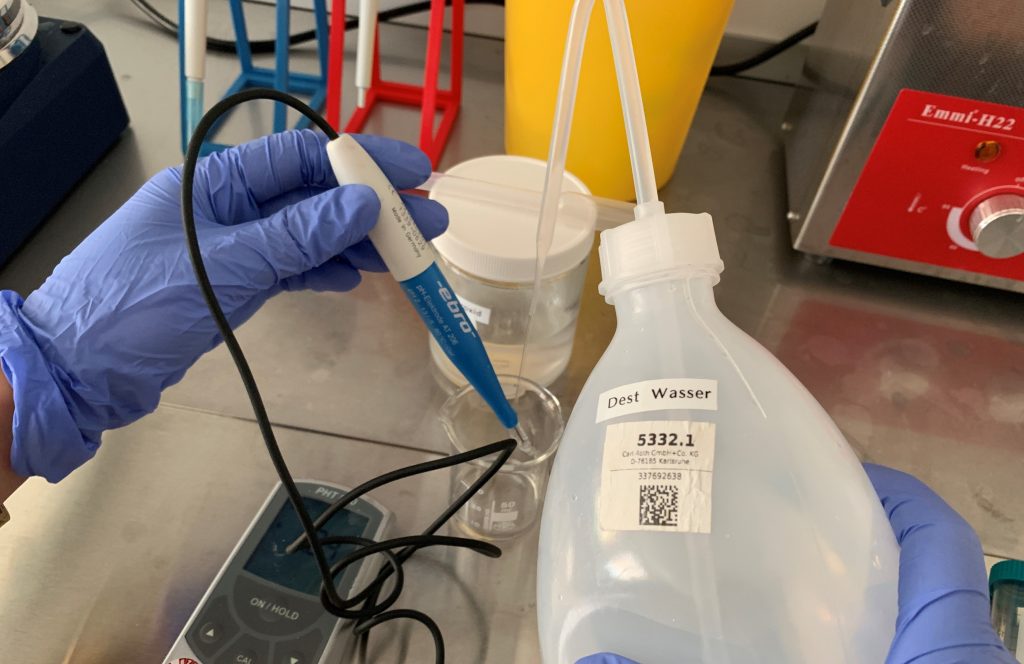

In addition to the mere concentration of tools and materials in one room, this has also made it possible to create more ergonomic and time-efficient workstations that quickly became popular with the team after they had been set up.
Our MDR/IVDR customers can be confident that we have quick access to a well-equipped, norm-compliant environment for our development services at all times.



Do you have questions about our services and equipment?
Then contact us at any time!
Since August 2019, velixX GmbH’s quality management system has been certified according to the latest ISO standard for medical technology development – and has now been newly recertified in August 2022.
During the recertification audit, which took place on August 11th and 12th, the QM system of velixX GmbH was again certified as meeting all requirements of the standard.
“Quality management is a matter of course in medical technology – and for us at velixX it was clear that we would also have our quality management system certified,” emphasizes Managing Director Manfred Augstein. “Quality management does not depend on the size of the company – and we are pleased that we have once again been formally certified as working with medical technology-compliant processes.”
There is no conflict between the organization and the management of modern development processes and good quality management. On the contrary. For the customers of velixX GmbH, the QM certificate is further proof of the reliability and the high quality of results of the services offered.
“Our customers rely not only on our know-how and creative approach to problem solving but also on the reliability and high quality of all our services.”
Long awaited. Exciting from start to finish. And already successful!
It has only been a month since MedtecLIVE in Stuttgart, but the first impression after quite an exhausting week has already been confirmed for velixX.
Almost the entire team was on site. All our engineers were directly approachable and accordingly, many in-depth conversations were possibly. What caused astonishment among some of our new contacts is a matter of course for us:
We are a small team in a highly specialized industry and offer very individual and complex services. Direct communication without long detours is crucial for us. Not only in an initial conversation, but throughout the entire development process.
We are delighted with the great interest shown in us and the numerous new contacts we were able to make in Stuttgart. But we are just as happy about the personal reunions with current and former customers and partners.
See you again in Nuremberg for MedtecLIVE 2023!
Video: © NürnbergMesse GmbH – thank you!
We have had to go withtout trade fairs and conferences for far too long and thus without personal meetings. Now things are really kicking off again with MedtecLIVE in Stuttgart.
Would you like to visit us at booth 10-337? Then contact us for tickets and to make an appointment at mail@velixx.com.
In the meantime, you can read an interview with our CEO Manfred Augstein on the occasion of MedtecLIVE here:
MedtecLIVE with T4M: velixX GmbH is a development company of the “next generation” in medical technology – what does that mean and which topics are relevant?
Manfred Augstein: We are management consultants, technology consultants, and also technology developers. We advise on questions of technical feasibility, business plans and also on basic market positioning. One particular point of focus is regulatory affairs concerning the new IVDR or the MDR. In the process, customers are advised and supported so that they get everything they need on the table. In addition, sustainability also plays a role: how to minimise waste and reduce the carbon footprint in new products, e.g. by using alternative materials or through miniaturisation – we actively support this process.
MedtecLIVE with T4M: Who are your customers? Start-ups, global players or medium-sized companies?
Manfred Augstein: We cover a broad spectrum, from start-ups to multinationals. We do initial consultations on technical implementability, check feasibility, develop products, even for global players who need support in certain niches. But we always focus on where the biggest technical problems or challenges can be found.
MedtecLIVE with T4M: velixX GmbH is a management consultant, technology consultant and technology developer. What does that involve?
Manfred Augstein: Yes, there is a quite a bit involved, and it is hardly possible to fulfil all this as a single company. We see ourselves more as a spider in a web. Our own focus is on microsystems and microfluidic parts. For everything that goes beyond that, we have a large network of competent partners who can, for example, implement serial production or develop software. With this network, we can quickly bring together very flexible teams. We do a system analysis, a problem analysis and offer an optimised solution concept.
MedtecLIVE with T4M: What does such a consulting process look like?
Manfred Augstein: The typical case is like a project we have just started. The company has a few inventors who have taken out a patent on paper for an idea. They then come to us and say: ‘Can you take a look at whether this is even possible?’ And then the project starts with a requirements analysis: ‘what should the product be able to do, what are the general conditions and so on’. Then the functional structure is analysed, followed by one or two workshops to analyse the critical points. A technical analysis follows; is there already data, what needs to be done next? This is followed by detailed planning of the steps needed to find the best solution.
That leaves us with concepts for prototypes which are implemented with the appropriate partners in the network. In working with us, customers can easily scale how comprehensively we take care of the project. They can leave it entirely in our hands and say I only want to see the end product, or they can hand over partial tasks to us. That is very flexible and very individual. Sometimes even large corporations come to us with a product that is almost finished but still needs some fine-tuning. Other customers say, ‘Here’s our idea, our wish, go for it’. We always focus on what the customer wants or needs.
MedtecLIVE with T4M: Why medical technology? What makes this sector so exciting for management consultants?
Manfred Augstein: This industry is the most exciting of all because it really does bring immense benefits to people and without technology, modern medicine would be unthinkable. On the other hand, it is super exciting technologically, because many disciplines come together: medicine, biochemical processes, organic and medical materials, optics, and microsystem solutions. Many elements have to merge, which makes the systemic interfaces in particular very challenging – that’s exactly where we advise.
MedtecLIVE with T4M: What makes your company unique?
Manfred Augstein: The fact that with our small team we can tackle complexities for which other companies need very large and complex organisations. We have broad knowledge, consulting skills, and competent partners. We prefer to work on projects with many functions in the smallest dimensions – that is our speciality. We take very different perspectives, work efficiently, have open, good people who are also wired to think creatively and in a complex way. That is a learning from my many years of professional experience.
MedtecLIVE with T4M: What is the benefit for society?
Manfred Augstein: As a rule, the products we develop help with faster or better diagnosis, especially in our focus area, point-of-care diagnostics. We want to increase reliability and quality, and this is always linked to the goal of producing less waste, making parts smaller and developing more sustainable products.
MedtecLIVE with T4M: What about the market – how will it develop?
Manfred Augstein: There is a lot of potential in this market because medical technology is developing rapidly. The last two years in particular have shown that we have to become even more agile and much faster in order to be able to deal with something like pandemics more efficiently. But most of the time we face more legal problems than technical ones. There is also a need to catch up, for example in digitalisation or in optimising the interfaces between diagnosis and therapy. We have just come through a period in which we had to wait a year until sufficient COVID-19 rapid tests were available. We can’t let something like that happen again.
MedtecLIVE with T4M: One last question: What is your favourite project?
Manfred Augstein: My favourite projects concern the miniaturisation and acceleration of rapid diagnostics. We have a project right now to quickly detect methanol poisoning. This is given far too little focus in the world: the ability to help quickly on the spot, with little effort. Sure, it could be that this also requires a novel approach. But that’s what makes it my favourite project right now.
All this is hardly conceivable without disposables. And that is exactly why it is so important for us at velixX to push this area of medical technology.
We are all familiar with the mountains of rapid tests that are currently being created all over the world. This has shown how important our focus on sustainability is where disposables are concerned. From the planning stages to the final disposal.
We use our customers’ new ideas as a starting point for development: from requirements to technical feasibility to an initial serial production.
Because the members of our team bring a wide range of experience from different disciplines to the table, it is not difficult for us to understand even complex interdependencies and interactions down to the last detail.
Combining many functions in the smallest dimensions is one of our strengths. And is, of course, also a good way to reduce material use and waste.
What does that look like? From the microfluidic cartridge to the complete lab-on-chip – many functions can be combined in very small plastic parts.
Does your task require a complex solution in a very small space? Then you have come to the right place.
We at velixX wish you a peaceful and above all healthy holiday season!
We look forward to seeing you again in 2022.
MEDICA and COMPAMED offer so many opportunities for exciting discussions that the four days have gone by in a flash.
We were able to revisit with long-standing customers and partners, but also got to meet numerous new people.
If we’ve piqued your interest and you would like to get to know us better: Browse through our site and contact us by phone or at mail@velixx.com.
We will be happy to talk to you about your ideas and projects and how we can join forces to make them successful.

We freely admit it: we’ve missed the personal encounters. Even though our industry in particular has had more important things to do in the past two years than attend trade shows.
In addition, the existing networks and collaborations in medical technology have played a major role in ensuring that, even without face-to-face meetings, diagnostic procedures and therapeutic approaches have been developed and advanced at high speed to help cope with the pandemic.
We would like to take this opportunity to thank our colleagues in the industry for their efforts. They have all worked under difficult conditions, doing overtime around the clock, and certainly not just “doing their job.”
Some things have been left undone in the past two years. But in other areas, one milestone after the other has been achieved under high pressure. And we have all learned a lot.
We at velixX are pleased that we now have the opportunity at MEDICA and COMPAMED 2021 in Düsseldorf to once again enter into creative exchange not only with the many existing partners and customers, but also with the entire industry.
If you have a new idea or need new input for an existing project – if you are looking for a sounding board or advice on the best cooperation partners:
Contact us – preferably at mail@velixx.com – and make an appointment to meet us at MEDICA or COMPAMED.

This is what defines our diagnostic systems.
velixX builds bridges between the worlds, translates the languages, and puts critical components together.
Biology and engineering, doctors and computer scientists, medicine and physics, man and machine, mass production and precision, quality managers and developers.
This may seem impossible at first, but finding the limit of what is feasible is precisely one of our tasks.
We are a small team of highly specialized engineers who are well connected in all subject areas.
When velixX celebrated its 10th anniversary in 2020, the company, like the entire industry, was busy with other issues. But in October 2021 the “next generation” development company celebrates 10 successful years at the Mannheim-Seckenheim location. And they are taking the opportunity for a look back.
This is how founder and CEO Manfred Augstein sums up the strengths of his company. His approach? No sluggish, all-encompassing company, but the coordination of a network of the best available development partners.

“The dynamic interaction of our development teams with the experts required in each case enables us to work on projects with the necessary flexibility and with large capacity reserves,” emphasizes Augstein, who previously worked in various functions in the diagnostics industry for 20 years. “Solid and long-term development partnerships form the core of our corporate concept.”
Thanks to quick, direct communication and the large variety of specialists, velixX is able to work quickly and in an interdisciplinary manner. Where medicine, biology and technology collide, velixX really gets going and builds bridges between the different spheres. Wherever the limits of what is feasible have apparently been reached, the small team of highly specialized and excellently networked engineers brings together critical components.
Mutual professional understanding is the prerequisite for systemic thinking and acting. Always focused on the solution and always committed to the best result. Flat hierarchical structures and team members working in their strong fields make for an uncomplicated and well thought-out way of working. Short decision-making channels and great freedom of action for each individual ensure fast and pragmatic action. To ensure that customers get real value for money, the entire team is keen to waste little time.
The paths are short, the partners respond quickly, and the results are goal-oriented.
Since quality is a key issue and an absolute must in the field of medical devices, the company’s quality management system is, of course, certified in accordance with ISO 13485 since August 2019.
Pragmatism, precision and quality go hand in hand for velixX . Because safety, reliability and usability are expected of healthcare products, velixX has made it its goal to clear this path for its customers and development partners. Over the past 11 years and in the future.
“We are using the company anniversary as an opportunity to take a closer look at our services and to focus once again on what distinguishes our brand from others,” explains Manfred Augstein, who plans to make up for the missed anniversary celebration in the coming year. “We welcome feedback from all those who have worked with us in recent years – and we invite those who have not yet met us to get in touch and to get to know us.”
Let’s continue with the multimedia insight into our medical device development: We develop medical technology and in-vitro diagnostics products in collaboration with highly innovative customers. We find solutions for all kinds of technical challenges.
Our first Company Video is now live.
We are happy to finally present our company in moving pictures!
Feel free to contact us for more information: mail@velixx.com
Since August 2019 our quality management system is certified according to the latest ISO standard for medical device development.
The mdc medical device certification GmbH confirms, that the QM-System from velixX corresponds to ISO 13485:2016 standard in all points.

Just in time before the 9th anniversary of the company’s foundation, the quality management system of velixX GmbH was certified according to ISO 13485:2016.
During the certification audit carried out on 22/23 July 2019, the velixX QM system was certified to meet all requirements of the standard. The audit report certified that all processes fully comply with the requirements of the standard without any deviation.
Based on this, the certification committee of mdc medical device certification GmbH issued the certificate to velixX GmbH.
velixX is very proud to be able to show that quality management does not depend on the size of the company and that the company has thus also been formally certified to work with medical technology-compliant processes.
The organization and management of modern development processes do not contradict good quality management.
For the customers of velixX, this is a further proof of the reliability and high result quality of the services offered.

velixX offers technology consulting and system development for In-Vitro-Diagnostics and Medical Devices.
When faced with complex challenges at the interface of biology, disposable and device – we deliver fast and pragmatic support. Our services include Consulting – Fire Fighting – Feasibilities – Disposable Development – Device Development
Ask for your individual tickets and visit us!
This year again velixX will be present in hall 8A / J19 of the Compamed trade fair.
Together with selected WIN partners of the WILD Group we present our capabilities to support your success.
Product development in medical technology and in-vitro diagnostics, fast, efficient and target-oriented.
Learn more about the “next generation development” concept and the possibilities of a modern and agile product development.
Visit us and discuss with us and our partners about the possibilities to bring your projects to the goal even faster than today.
Discuss with us the ways to systematically and unerringly arrive at perfect technical solutions in a complex technical environment.
Visit us from 12.11. – 15.11. in Düsseldorf in Hall 8A / J19

velixX is present at the famous European Trade Show for Medical Technology Innovations.
Visit us and learn how our concept of “next generation development” can speed up your product development process.
Receive support just where you need it: Technology consulting, development support and any kind of disposable and device development.
We are looking forward to meeting you at our booth – H9 C18
Book your free ticket right now!
velixX will be present at Compamed in Düsseldorf from 14. – 16.11.2017.
We are happy to meet our business partners at the booth of Wild GmbH in hall 8A, J19
Together with our partners from Wild Group, the experts for optomechanical components and instruments, we will be able to host our partners and customers for any kind of meetings and project discussions. We will be present from Tuesday to Thursday continuously.
We are looking forward to interesting discussions and requests and are open for fixed appointments via mail@velixx.com.
velixX is proud to announce the colaboration with Orphan Diagnostics to develop the next generation of Point-of-Care Tests to detect Methanol Poisoning.
Our client, Orphan Diagnostics is a highly innovative norwegian company, which has the goal to fight against a global burden.
Poisoning due to mixing methanol with regular alcohol causes thousands of deaths, brain damage and blindness worldwide every year.
This affects people in all ages – children, adults and elderly – with the majority being young adults in employable ages.
And all over the world, the numbers of methanol poisoned people are increasing.
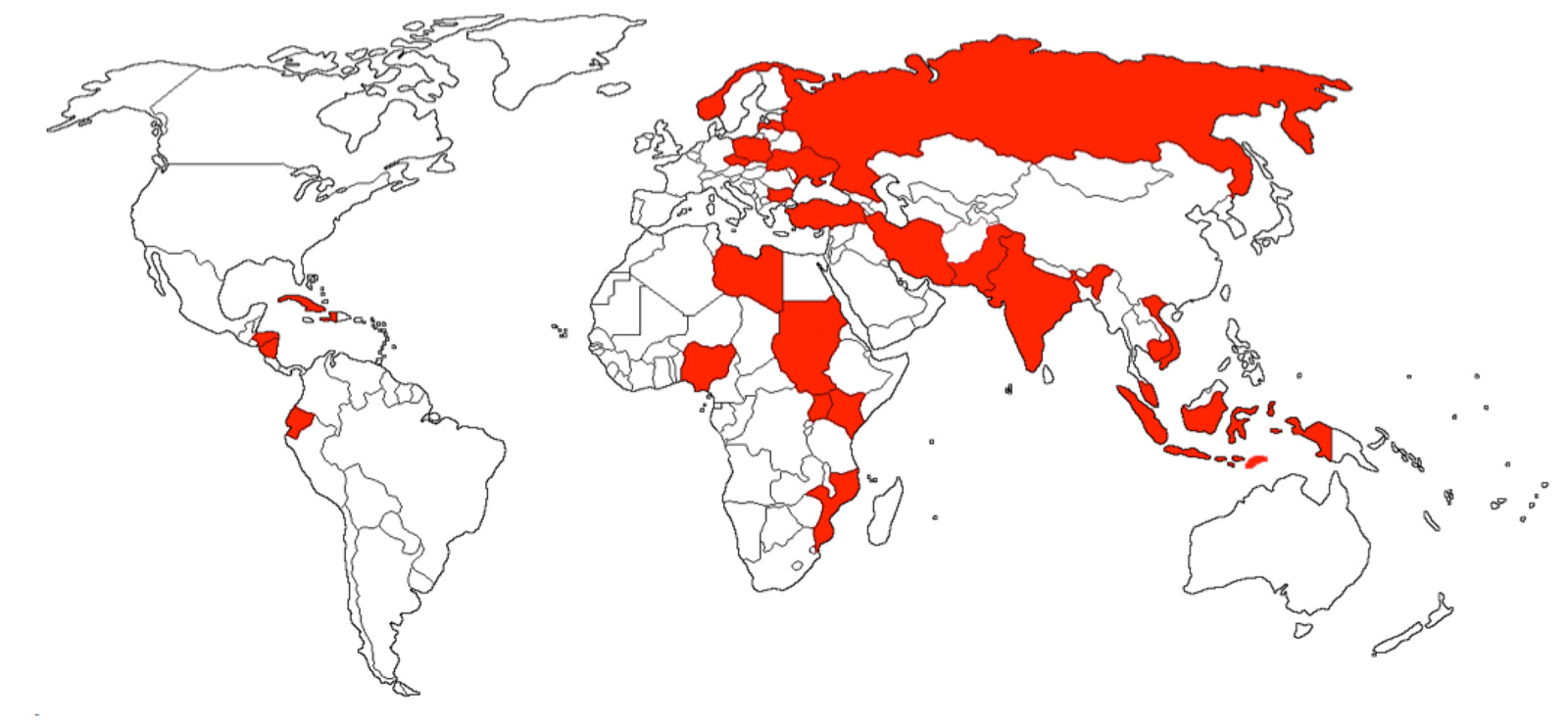
Effective treatment is available, but it requires a fast, reliable and simple diagnosis. Currently there are only complicated laboratory procedures available, namely specific chromatographic methods, or unspecific analysis like osmolality measurement.
In the environments where most of the injuries appear, these technologies are usually not available. Thus, simplification of the diagnostic process is warranted..
Exactly here the support of velixX gets valuable. Together with the excellent Toxilogists and Medical Experts from the University of Oslo, we support Orphan Diagnostics in developing a precise and reliable Point-of-Care Test.
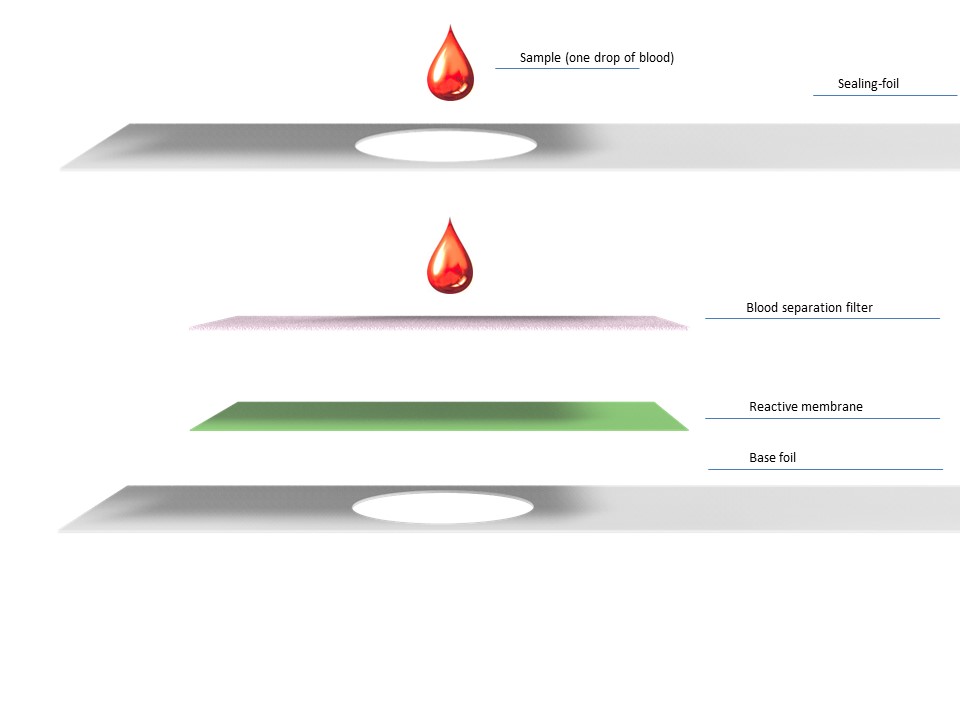
The MeTOX – Test is able to detect the formic acid (the toxic metabolite of the Methanol) semi-quantitatively in a small drop of whole-blood.
Therefor the experience of velixX with exactly these kind of system-interfaces, adds value to the project and ensures a reliable diagnosis.
The project is also supported by large international humanitarian organizations such as Doctors without Borders.
We are looking forward to a very promising and successful project for a better Health-Care all over the world,
together with
I would like to sincerely thank you for the lively response of our existing and especially our future customers and partners at the Medtec Europe in Stuttgart.
The discussions at our booth have given us a lot of fun this year, and it was a great pleasure for us to lead as many new project discussions as never before!
The velixX team was in top form for 3 days, so a very personal thanks to my sensational team – you have been great.
Now we are looking forward to many exciting new projects.

The velixX team at the famous medical device event MedTec Europe
As every year we are there with our booth

FREE VISITORS REGISTRATIONS:
REGISTER HERE

THE Health-Care Consulting network!
Fruitfull discussions – perfect people!

with R&D innovative award
VISIT US AT MEDTEC EUROPE IN STUTTGART – 12.-14.04.2015 – HALL 3 – E80
The velixX GmbH as a well-known problem solver in the field of in vitro diagnostics is looking forward to meeting you this year again at the Medtec Europe from 12 to 14.4.2016 in Stuttgart.
We can offer you all our skills and competencies in system development of medical devices and especially in the area of In-Vitro Diagnostics. Wherever you have real challenges in sample handling, disposable-development and -manufacturing or anywhere in the feasibility of technical solutions we will love it to support you.
Based on our long-term experience and our excellent network of experts we will find the appropriate solution for your topic.
Our goal is to save your money and your time.
We are looking forward to serve your individual needs – from consulting only up to complete development projects.
Let’s discuss it and visit us at our booth in hall 3 E80 – it is a pleasure to arrange an individual appointment with you.
On 11th March we will all meet at the MedTechDialog meeting, where we have the opportunity to visist the brand new CUBEX – Center!
The Core of our Medical-Device Cluster in Mannheim.
Main Topic of the meeting is “Smart Innovation” exctly out main topic – so we are looking forward to interesting discussions.
Invitation to MedTechDialog

Again we had the opportunity to join the 81st Annual Meeting of the German Cardiac Society together with our Cluster for Medical Technology Mannheim. Very interesting talk and discussion took place.

The second Festo Lab Automation Symposium was organised at Esslingen last week (on Thursday, 30 October 2014) in which velixX also participated.
At the symposium, Festo AG & Co. KG had invited several health industry experts from across the globe, to share their views and experiences on important topics affecting the health industry – such as increasing cost pressure and the consequently rising demand for rapid implementation of new molecular diagnostics strategies. It has led to a greater need for more efficient automation concepts and for quickly bringing them to the market. This constituted the key focus of the symposium.
Well known health experts from around the globe (including USA, UK, Austria, Germany, Italy) showcased their views and experiences in the field of laboratory process automation and demonstrated various approaches to solutions at the symposium.
The event was attended by members of velixX staff and Mr. Manfred Augstein (CEO, velixX GmbH). It provided an excellent opportunity for highly informative discussions on the topic and also for connecting with industry partners. There were exciting presentations on automation and optimisation in the field of Biomarkers, Biobanking, Personalised Medicine, actual practice and future applications during the event.
Insightful presentations and panel discussions in the morning were followed by a refreshing afternoon session where attendees could choose between a guided tour of the Festo factory, a seminar on Nano- and Bio-Engineering, or a visit to the World Café for further discussions on the topic of Personalised Medicine and Companion Diagnostics. An exhibition of current and future automation concepts from the health industry was also organised at the event, offering many other interesting first-hand insights in the field of lab automation.



The MedTechDialog in the Rhine-Neckar metropolitan region brings together industry experts interested in medical technology to discuss the latest trends and explore special topics. The recent MedTechDialog took place on September 9, 2014 in Mannheim, organized by the city of Mannheim and PWC (PricewaterhouseCoopers Aktiengesellschaft Wirtschaftsprüfungsgesellschaft). A stimulating exchange ensued on the theme of “recognizing and skillfully accessing public support programs for corporate and product development”. Attractive opportunities for funding and financing of R&D projects on the state, federal, and EU level were presented and discussed, a highly relevant topic for managers and decision-makers in the metropolitan area.
At the event, Mr. Manfred Augstein (CEO, velixX GmbH) presented the recent successful experience of velixX in acquiring project funding from the Central Innovation Program SME (Zentrales Innovationsprogramm Mittelstand, ZIM) for an upcoming in-house product development project (in cooperation with two other SMEs). The funding (in the range of 430 thousand euros) will be provided over a period of two years. Demonstrating with a practical example, Mr. Augstein described the funding process involved and how it offers great advantages for projects that may sometimes be considered risky due to their niche nature. Currently, velixX has two product development projects for which government funding has been approved.
We hope to see you at the next MedTechDialog on December 9, 2014 at Technoseum Mannheim.
